With the ongoing COVID-19 pandemic and climate crisis, now more than ever we see the need for more resilient and circular healthcare systems that can both minimise their contribution to climate change and adapt to the resulting health threats. It has been an extremely challenging year for the sector and there is still a long way to go before “returning to normal”, but the question remains - what do we want this “normal” to look like?
Single-use devices (SUDs) are a large part of the healthcare sector’s negative environmental impact - they contribute to the growing disposable culture that has emerged in the sector, generating more waste and greenhouse gas emissions. The significant quantity of single-use products used in healthcare has not only increased the sector’s waste, pollution, and environmental impact, but it has also increased healthcare expenditure and made supply chains vulnerable to disruption and fluctuations in demand.
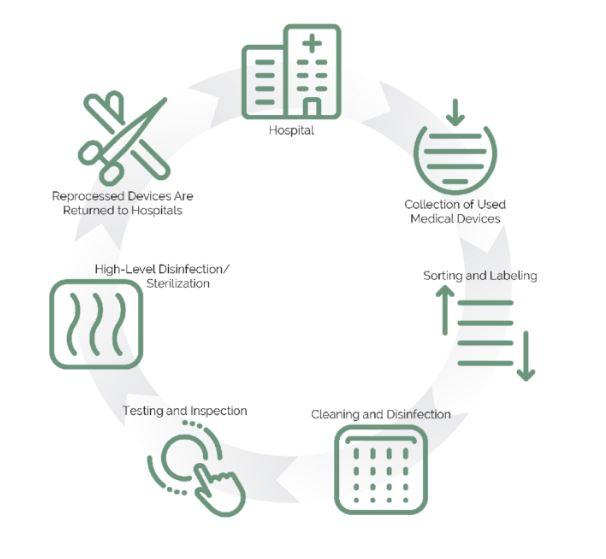
During the ongoing COVID-19 pandemic, particularly early on in 2020, healthcare facilities faced dramatic supply shortages, exacerbated by the single-use culture and complex supply chains. Many turned to the reuse and reprocessing of single-use respirators, face shields and surgical gowns. It is essential to build such resilience to mitigate the risk of future crises, including climate change - we must consider how products are used, reused, and processed at the end-of-life.
Read more: Sustainable healthcare waste management
Reprocessing and remanufacturing SUDs is therefore an opportunity for health systems to minimise waste and achieve sustainability goals. Working with reprocessors to safely collect and purchase reprocessed SUDs is one of the biggest opportunities for cost savings and waste reduction in the operating room and should be a core principle of sustainable procurement practices in healthcare. The National Health Service in England’s bold commitment to net-zero emissions by 2040 importantly includes the reuse of SUDs as one solution to address the significant carbon emissions generated throughout supply chains.
Read more: NHS England commits to net-zero emissions by 2040
The myths of single-use
The barriers of circularity in healthcare typically include misconceptions regarding infection prevention, which makes changing the behaviour of device users a challenge. There are also regulatory structures that encourage the proliferation of disposable medical devices, but the reuse/reprocessing of medical devices plays a key role in the roadmap to a circular healthcare economy.
There is strong evidence that reprocessed devices have a significantly lower environmental impact; the Life Cycle Analysis of a remanufactured electrophysiology catheter, for example, shows that the global greenhouse gas emission is reduced by 50.4% compared to a new product. The environmental impact is reduced further still with increased collection and reprocessing rates of catheters.
The regulatory situation of reprocessing in Europe
Current European regulations create incentives to make devices disposable. Last year, the European Commission released its long-awaited Common Specifications that set strict conditions for the reprocessing of single-use medical devices, and placed full product liability on the reprocessor. These specifications are just one part of the much larger EU Medical Device Regulation (MDR) which will fully apply from 26 May 2021 (already delayed one year due to COVID-19).
The MDR can support EU countries to reduce their medical waste and associated costs as well as build resilience into the healthcare supply chain - a win-win situation. But Member States must be proactive and act to take advantage of this - the MDR only allows for the reprocessing of SUDs if it is also permitted under national law and in compliance with Article 17 of the Regulation.
There are two options under Article 17:
- Hospitals can continue to reprocess in-house, but such reprocessing has to meet the Common Specifications.
- Members States may also “opt in” to allowing regulated, professional firms that fully meet medical device manufacturer requirements to carry out reprocessing of medical devices
It is important to understand that each EU country must admit one or both of the reprocessing options under the MDR, otherwise, single-use device reprocessing becomes illegal. We advise our members within the EU to contact their national authorities, who are responsible for implementing this Regulation, and inquire about the legal status of reprocessing. Across Europe, we encourage you to use the arguments laid out in this article to discuss with your national authorities why it is important to have the option of reprocessing SUDs.