Hazardous chemicals found in many medical devices, such as phthalates (especially DEHP) and Bisphenol A (BPA), pose direct health risks to patients. The evidence base for these risks are growing and healthcare facilities in Europe are looking to substitute potentially harmful medical devices.
The second edition of our Non-toxic Healthcare report, now available in Spanish, builds on the 2014 report and provides up to date evidence of the health risks posed by some medical devices as well as further substitution case studies and a new chapter exploring the use of plastics in healthcare.
Modern healthcare uses a wide range of plastic-based medical products to provide high quality and effective treatment to patients. As a consequence, high volumes of single-use plastic products and complex plastic composites are routinely used by the European healthcare sector and it is becoming increasingly important to understand the impact these materials have on human health.
Patients and healthcare professionals can be exposed to hazardous chemicals, including endocrine disrupting chemicals (EDCs), during medical procedures – a continuous, long-term, and low-level exposure to a mixture of different hazardous chemicals can cause or enhance adverse effects on both human health and the environment.
The Non-toxic Healthcare report also highlights relevant legislation and initiatives to reduce exposure in healthcare, such as the 2017 EU Medical Devices Regulation (MDR), which introduced provisions to further support a phase-out of endocrine disrupting chemicals (particularly phthalates) and carcinogenic, mutagenic and reprotoxic substances in medical devices. Originally set to come into force in 2020, this date was extended by 12 months, largely due to COVID-19, which means that the MDR will be fully applicable from next month - 26 May 2021.
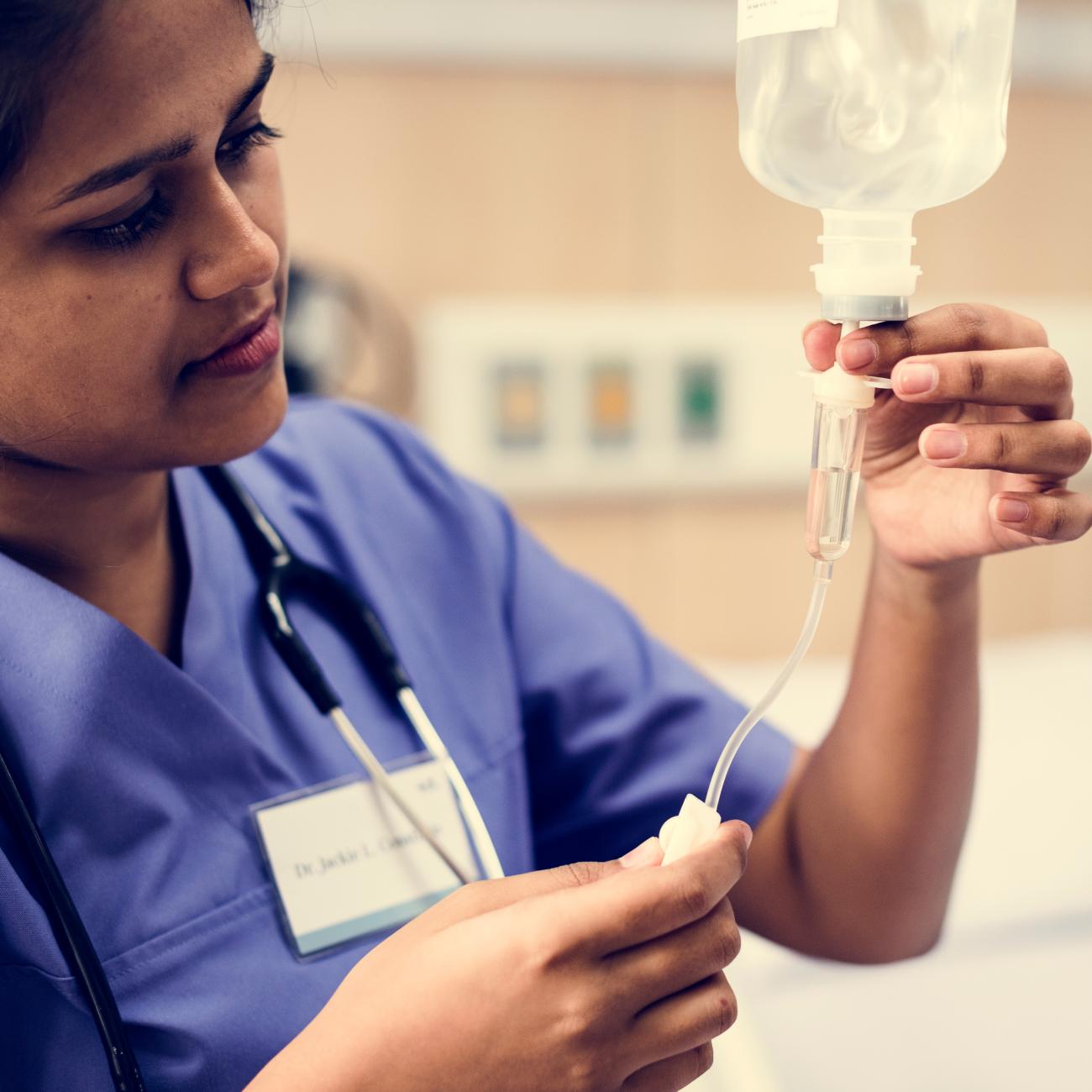
Safer, alternative medical devices already exist and are available for nearly all product categories; we can protect vulnerable patients (including the unborn) from harmful exposures by insisting on PVC-free, DEHP-free, and BPA-free products.
Further development of safer medical devices by manufacturers and greater demand from healthcare professionals, could see the authorisation of DEHP in medical devices being denied when safer alternatives are available and ultimately contribute to a complete transition away from DEHP and BPA.
- Non-toxic Healthcare: Alternatives to Phthalates and Bisphenol A in Medical Devices (HCWH Europe, 2014)
- Non-toxic Healthcare: Alternatives to Phthalates and Bisphenol A in Medical Devices edition II (HCWH Europe, 2019) [EN] [ES]

